
Our purpose
To improve life during and beyond sedation
Sedana Medical is a pioneer medtech and pharmaceutical company dedicated to making inhaled sedation a standard therapy in intensive care.
More about usBringing inhaled sedation to intensive care
We are proud to introduce inhaled sedation to intensive care, a paradigm shift in the care of critically ill patients.
Through the results obtained in the pivotal study with Sedaconda (isoflurane) and Sedaconda ACD*, we have laid the foundation for inhaled sedation in intensive care.
By this, we have also taken a major step closer to our vision – to make inhaled sedation a standard therapy in intensive care.
*formerly known as AnaConDa

Science & Clinical Research
We pave the way for a paradigm shift in sedation in intensive care through knowledge sharing and clinical research.
Sedasource is an online resource for healthcare professionals, providing scientific and clinical material on the sedation of critically ill patients.
Our research aims at a greater understanding of and increased knowledge about inhaled sedation, leading to medical advances for the benefit of patients in intensive care.
We also believe in working in partnerships – through various initiatives and research projects – that can lead to continued development and meaningful improvement for both patients and the healthcare system.
Press Releases
Sedana Medical AB's Interim report January-March 2024
Regulatory
Off to a strong start with record sales in Q1
Sedana Medical completes patient recruitment for INSPiRE-ICU 1 clinical trial in the US
Non-regulatory
Sedana Medical AB (publ) announces that all 235 randomized patients have been recruited for its INSP…
Our products
Explore our broad portfolio of products for the administration of inhaled anaesthetics.

Sedaconda ACD-S
Sedaconda ACD-S is a medical device which enables the administration of volatile anaesthetics (isofl…

FlurAbsorb-S
FlurAbsorb is an active carbon filter, developed by Sedana Medical to capture waste anaesthetic gase…
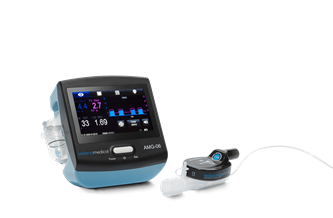
AMG-06 Gas Monitor
AMG-06 Gas Monitor is intended for continuous non-invasive sidestream monitoring of CO2 & anaest…


